ISSUE1697
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Discuss the new warning about the use of denosumab (Prolia) in patients with chronic kidney disease.
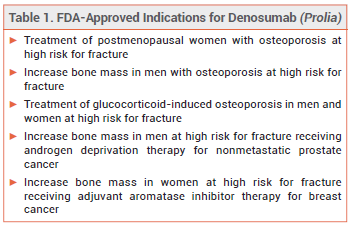
The FDA is requiring a boxed warning in the label of denosumab (Prolia – Amgen)1, a monoclonal antibody that inhibits osteoclasts, about an increased risk of severe hypocalcemia in patients with advanced chronic kidney disease (CKD; eGFR <30 mL/min/1.73 m2), particularly those on dialysis.2 FDA-approved indications for Prolia are listed in Table 1.
Denosumab is also available as Xgeva for prevention of skeletal-related events or treatment of hypercalcemia in patients with malignancies. The warning has not been added to the Xgeva label.
An FDA analysis of studies from the Centers for Medicare & Medicaid Services found that use of Prolia in patients with advanced CKD was associated with a significant increase in the risk of developing severe hypocalcemia, compared to use of bisphosphonates. In 2804 dialysis-dependent patients treated for osteoporosis, the incidence of severe hypocalcemia was 41.1% with denosumab, compared to 2.0% with oral bisphosphonates. Prolia is given subcutaneously once every 6 months. Severe hypocalcemia, which generally occurred 2-10 weeks after each injection of the drug, can lead to muscle spasms, seizures, cardiac arrhythmias, and death.2,3
In patients with advanced CKD, pre-existing hypocalcemia should be corrected before starting Prolia and serum calcium levels should be monitored weekly for the first month of treatment with the drug and monthly thereafter. Adequate calcium and vitamin D supplementation can decrease the risk of hypocalcemia.
- Drugs for postmenopausal osteoporosis. Med Lett Drugs Ther 2020; 62:105.
- FDA Drug Safety Communication. FDA adds boxed warning for increased risk of severe hypocalcemia in patients with advanced chronic kidney disease taking osteoporosis medicine Prolia (denosumab). January 19, 2024. Available at: https://bit.ly/3I7zGjb. Accessed February 14, 2024.
- ST Bird et al. Severe hypocalcemia with denosumab among older female dialysis-dependent patients. JAMA 2024; 331:491. doi:10.1001/jama.2023.28239