ISSUE1673
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Discuss the interchangeability status of the FDA-approved biosimilars of Lantus.
Insulin glargine-aglr (Rezvoglar – Lilly), which was approved by the FDA as a biosimilar to the reference product Lantus in 2021 and received interchangeability status with Lantus in 2022, will become available in the US on April 1, 2023. It is the second biosimilar insulin product to be designated as interchangeable with Lantus; Semglee was the first.1 Rezvoglar did not receive interchangeability status with Lantus at the time of its initial approval because the manufacturer of Semglee had exclusivity for 12 months.

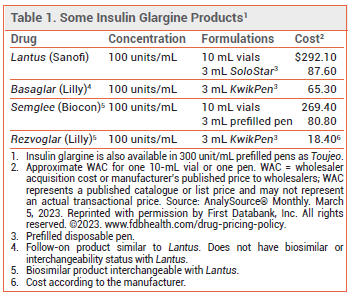
A biosimilar is a biologic product that is highly similar in composition, strength, and biological properties to the FDA-approved reference product and has no clinically meaningful differences. No new clinical studies were required by the FDA to support approval of interchangeability status. The decision to designate Rezvoglar as an interchangeable product was based on a lack of clinically meaningful differences in safety, purity, and potency between Rezvoglar and Lantus.2
Basaglar is an insulin glargine product that is similar to Lantus in composition, strength, and biological properties and has produced comparable clinical results, but it does not have biosimilar or interchangeability status.3
According to federal law, an interchangeable product can be substituted for the reference product by the pharmacist without permission from the prescriber. Some states require the pharmacist to notify the prescriber and/or patient before making the substitution; currently four states (AL, IN, SC, and WA) restrict interchangeability entirely.4
- In brief: Semglee – insulin glargine interchangeable with Lantus. Med Lett Drugs Ther 2021; 63:159.
- FDA Guidance Document. Clinical immunogenicity considerations for biosimilar and interchangeable insulin products. November 2019. Available at: https://bit.ly/2VYOJ9I. Accessed March 16, 2023.
- Another insulin glargine (Basaglar) for diabetes. Med Lett Drugs Ther 2017; 59:3.
- S Jeremias. Part 2: for patients and employers, 2023 means a changed landscape. AJMC The Center for Biosimilars. September 13, 2022. Available at: https://bit.ly/3JL20JP. Accessed March 16, 2023.
