ISSUE1641
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Michael Viscusi, Pharm.D., Associate Editor: no disclosure or potential conflict of interest to report
- Review the efficacy and safety of tixagevimab and cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19.
Updated: January 12, 2022
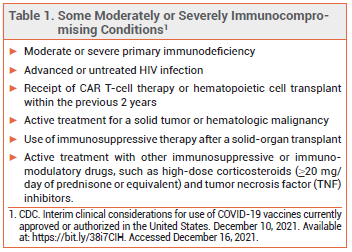
The FDA has issued an Emergency Use Authorization (EUA) for the investigational long-acting monoclonal antibodies tixagevimab and cilgavimab (Evusheld – AstraZeneca) to be administered concomitantly by IM injection for pre-exposure prophylaxis of COVID-19 in persons ≥12 years old who weigh ≥40 kg and have either a history of severe allergy that prevents their vaccination against COVID-19 or moderate or severe immune compromise (see Table 1). They are the first drugs to be authorized by the FDA for this indication.1 Two other pairs of antibodies, bamlanivimab plus etesevimab (Lilly) and casirivimab plus imdevimab (REGEN-COV), are authorized for post-exposure prophylaxis of COVID-19.2,3

IMMUNE COMPROMISE — Immunocompromised persons are more likely to have an inadequate antibody response to COVID-19 vaccination and severe breakthrough infection requiring hospitalization than healthy persons.4 Those ≥5 years old should generally receive a 3-dose primary series of an mRNA-based COVID-19 vaccine (Pfizer/BioNTech or, if ≥18 years old, Moderna) and those ≥12 years old should also receive a booster dose ≥5 months after the last primary dose.5-8 Booster doses are not currently recommended for persons <12 years old.
SEVERE ALLERGY — COVID-19 vaccines are contraindicated for use in persons with a history of severe allergic reaction (e.g., anaphylaxis) to the vaccine or any of its components. Patients who have a severe allergic reaction to one mRNA-based COVID-19 vaccine should not receive the other.5
PHARMACOLOGY — Tixagevimab and cilgavimab bind to non-overlapping portions of the SARS-CoV-2 spike protein, preventing the virus from interacting with the human ACE2 receptor. They are catabolized slowly; in pharmacokinetic studies, the mean half-lives of tixagevimab and cilgavimab were 87.9 and 82.9 days, respectively.9
CLINICAL STUDIES — Issuance of the EUA was based on the results of an unpublished double-blind trial (PROVENT; summarized in the FDA Fact Sheet) in 5172 adults who were not vaccinated against COVID-19 and at elevated risk because of their age (≥60 years), a comorbidity (e.g., obesity, COPD, immune compromise, history of severe/serious adverse reaction to any FDA-licensed vaccine), or their residential or occupational status. Subjects were randomized 2:1 to receive either 150 mg of tixagevimab plus 150 mg of cilgavimab IM or placebo.
In the primary efficacy analysis (median follow-up 83 days), symptomatic COVID-19, the primary endpoint, occurred in 8 patients (0.2%) who received the antibodies and in 17 (1.0%) who received placebo (HR 0.23 [95% CI 0.10-0.54]). Similar results were observed in a post-hoc analysis with a median follow-up of 6.5 months (0.3% vs 1.8%; HR 0.17 [95% CI 0.09-0.34]). There were no severe or critical COVID-19 events in the antibody group compared to 5 in the placebo group.9
ADVERSE EFFECTS — The most common adverse effects of tixagevimab plus cilgavimab in PROVENT were headache (6%) and fatigue (4%). Rates of overall and serious adverse events in the antibody and placebo groups were similar.
In the post-hoc analysis of PROVENT, the incidence of serious cardiac adverse events (e.g., myocardial infarction, cardiac failure, arrhythmia) was higher in the antibody group than in the placebo group (0.6% vs 0.2%). One person who received the antibodies died of a myocardial infarction. There was no clear temporal relationship between antibody administration and cardiac adverse events.1,9
VARIANTS — Tixagevimab plus cilgavimab is fully active against the Delta variant of SARS-CoV-2. The combination has somewhat decreased neutralizing activity in vitro against the Omicron variant (by 12- to 30-fold vs the ancestral virus); the clinical significance of this difference remains to be determined. The Omicron variant was not prevalent during clinical trials of Evusheld.9,10
DOSAGE AND ADMINISTRATION — Evusheld is supplied in cartons that contain one 150 mg/1.5 mL vial of tixagevimab and one 150 mg/1.5 mL vial of cilgavimab. The vials should be refrigerated before use. The recommended dosage is 150 mg of each antibody administered as consecutive IM injections into separate sites (preferably one in each gluteal muscle). Patients should be monitored for at least 1 hour after administration. Tixagevimab and cilgavimab can be given once every 6 months while SARS-CoV-2 is in circulation. The antibodies should not be used for treatment or post-exposure prophylaxis of COVID-19, or within 2 weeks after administration of a COVID-19 vaccine.9
CONCLUSION — The FDA has authorized concomitant use of the monoclonal antibodies tixagevimab and cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19 in patients who cannot be vaccinated against COVID-19 because of severe allergy or may not benefit fully from vaccination because of immune compromise. In a double-blind trial, one-time IM administration of the antibodies decreased the incidence of symptomatic COVID-19 compared to placebo in at-risk adults for 6 months. Tixagevimab and cilgavimab can be administered to eligible patients every 6 months while SARS-CoV-2 is in circulation.
Additional Content Available:
COVID-19 Vaccine Dosing Recommendations and Comparison Chart
COVID-19 Vaccine Comparison Chart
- FDA News Release. Coronavirus (COVID-19) update: FDA authorizes new long-acting monoclonal antibodies for pre-exposure prevention of COVID-19 in certain individuals. December 8, 2021. Available at: https://bit.ly/3saed1U. Accessed December 16, 2021.
- Bamlanivimab and etesevimab for post-exposure prophylaxis of COVID-19. Med Lett Drugs Ther 2021; 63:163.
- Casirivimab and imdevimab (REGEN-COV) for post-exposure prophylaxis of COVID-19. Med Lett Drugs Ther 2021; 63:130.
- K Dooling. Evidence to recommendations framework: an additional dose of mRNA COVID-19 vaccine following a primary series in immunocompromised people. Advisory Committee on Immunization Practices meeting, August 13, 2021. Available at: https://bit.ly/2Uqqy3H. Accessed December 16, 2021.
- CDC. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. December 10, 2021. Available at: https://bit.ly/38i7CIH. Accessed December 16, 2021.
- In brief: Third dose of mRNA-based COVID-19 vaccines for immunocompromised persons. Med Lett Drugs Ther 2021; 63:145.
- Booster doses of COVID-19 vaccines. Med Lett Drugs Ther 2021; 63:186.
- FDA News Release. Coronavirus (COVID-19) update: FDA expands eligibility for Pfizer-BioNTech COVID-19 booster dose to 16- and 17-year-olds. December 9, 2021. Available at: https://bit.ly/3GClETN. Accessed December 16, 2021.
- FDA. Fact sheet for health care providers: Emergency Use Authorization for Evusheld (tixagevimab co-packaged with cilgavimab). December 2021. Available at: https://bit.ly/3IWpQjg. Accessed December 21, 2021.
- AstraZeneca. Evusheld long-acting antibody combination retains neutralising activity against Omicron variant in independent FDA study. December 16, 2021. Available at: https://bit.ly/3Jluk3d. Accessed December 21, 2021.