ISSUE1633
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Michael Viscusi, Pharm.D., Associate Editor: no disclosure or potential conflict of interest to report
- Discuss the FDA's recommendation for a third dose of the mRNA COVID-19 vaccines in immunocompromised persons.
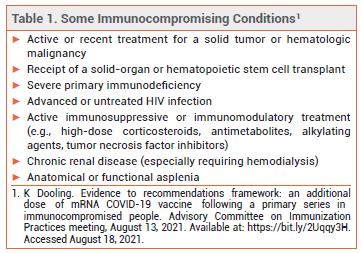
The FDA has expanded the Emergency Use Authorizations (EUAs) for the mRNA-based COVID-19 vaccines manufactured by Pfizer/BioNTech (Comirnaty) and Moderna (Spikevax) to include administration of a third dose in persons ≥12 years old (Pfizer/BioNTech) or ≥18 years old (Moderna) who have undergone solid organ transplantation or have a condition that compromises the immune system to a similar extent (see Table 1).1
Immunocompromised persons are more likely than healthy individuals to have an inadequate antibody response to COVID-19 vaccination, breakthrough SARS-CoV-2 infection, and severe COVID-19 requiring hospitalization. About 2.7% of adults in the US are considered immunocompromised, but 40-44% of hospitalizations for breakthrough COVID-19 cases have occurred in such persons.2 In a study in 658 solid organ transplant recipients who received 2 doses of an mRNA-based vaccine, anti-SARS-CoV-2 antibodies were detectable in only 54% of persons 28-31 days after the second dose.3
Several clinical studies have demonstrated that the immunogenicity of mRNA-based COVID-19 vaccines in immunocompromised persons is increased with administration of a third dose. In a double-blind trial in 120 organ transplant recipients, median antibody, T-cell, and virus neutralization levels were significantly higher in persons who received 3 doses of the Moderna vaccine (at 0, 1, and 3 months) than in those who received 2 doses.4 In 5 cohort studies, administration of a third dose of an mRNA-based vaccine to a total of 112 organ transplant recipients or hemodialysis patients who tested negative for anti-SARS-CoV-2 antibodies following their second dose resulted in seroconversion rates ranging from 33% to 50%.2 Adverse effects with a third vaccine dose in immunocompromised persons have been similar to those observed with the first two doses.5
Immunocompromised persons who have received two doses of the Pfizer/BioNTech or Moderna vaccine can now receive a third dose of the same vaccine at least ≥28 days after their second dose.6,7 The FDA has not authorized to date the use of additional doses of any COVID-19 vaccine in immunocompromised persons who received the Johnson & Johnson (Janssen) adenovirus-based vaccine.
ADDITIONAL NOTE:
On August 18, 2021, the US Department of Health and Human Services announced plans to offer a third dose of the Pfizer/BioNTech and Moderna COVID-19 vaccines to all persons in whom their use is authorized beginning the week of September 20, 2021, pending FDA and CDC review. The third dose would be administered ≥8 months after the second. More information can be found at: https://bit.ly/3yTxZPX.
- FDA News Release. Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals. August 12, 2021. Available at: https://bit.ly/3AM8QHq. Accessed August 18, 2021.
- K Dooling. Evidence to recommendations framework: an additional dose of mRNA COVID-19 vaccine following a primary series in immunocompromised people. Advisory Committee on Immunization Practices meeting, August 13, 2021. Available at: https://bit.ly/2Uqqy3H. Accessed August 18, 2021.
- BJ Boyarsky et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021; 325:2204.
- VG Hall et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med 2021 August 11 (epub).
- M Espi et al. Justification, safety, and efficacy of a third dose of mRNA vaccine in maintenance hemodialysis patients: a prospective observational study. MedRxiv 2021 July 6 (preprint). Available at: https://bit.l/3snU1bd. Accessed August 18, 2021.
- FDA. Fact sheet for health care providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). August 23, 2021. Available at: https://bit.ly/37fX1NG. Accessed September 7, 2021.
- FDA. Fact sheet for healthcare providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). August 27, 2021. Available at: https://bit.ly/3nosylA. Accessed September 7, 2021.