ISSUE1690
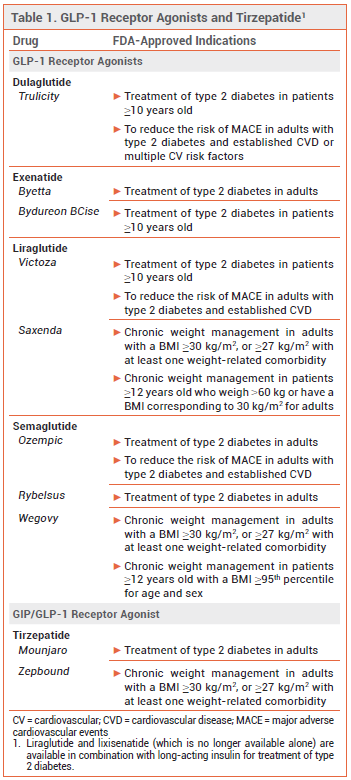
Glucagon-like peptide-1 (GLP-1) receptor agonists and the dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 receptor agonist tirzepatide (Mounjaro) are widely prescribed for treatment of type 2 diabetes and weight management (see Table 1), but they delay gastric emptying and commonly cause nausea and vomiting.1 Gastroparesis and bowel obstruction (ileus) have also been reported with their use.
ILEUS — The package inserts of all GLP-1 receptor agonists and tirzepatide have been updated to include postmarketing reports of ileus associated with their use. According to the FDA, the additions were based on 90 postmarketing cases of ileus reported to the FDA Adverse Event Reporting System (FAERS). Long-term randomized, controlled trials evaluating the risk of ileus with these drugs are lacking.
Possible Mechanism – In rodent studies, use of GLP-1 receptor agonists has been associated with increased intestinal length, weight, and villus height, which could reduce motility. Whether similar effects occur with use of these drugs in humans has not been established.2
EFFECT ON ORAL DRUGS — The delay in gastric emptying caused by GLP-1 receptor agonists and tirzepatide can alter the absorption and efficacy of orally administered drugs, including hormonal contraceptives. The labels of both tirzepatide products recommend that women taking oral hormonal contraceptives switch to a non-oral contraceptive method or add a barrier method of contraception for 4 weeks after starting tirzepatide and for 4 weeks after each dose escalation.
PREOPERATIVE MANAGEMENT — Delayed gastric emptying can increase the risk of regurgitation and aspiration of stomach contents during general anesthesia. Due to these concerns, the American Society of Anesthesiologists recommends withholding GLP-1 receptor agonists on the day of elective surgery for those that are administered daily, or for one week prior to the procedure if the patient is on a once-weekly formulation.3 Some diabetologists argue that the evidence supporting these recommendations is limited and that the risks of withholding a once-weekly administered GLP-1 receptor agonist or tirzepatide in a patient with type 2 diabetes and/or cardiovascular risk outweighs the potential benefits.4
- Drugs for type 2 diabetes. Med Lett Drugs Ther 2022; 64:177.
- J Lu et al. A potentially serious adverse effect of GLP-1 receptor agonists. Acta Pharm Sin B 2023; 13:2291. doi:10.1016/j.apsb.2023.02.020
- GP Joshi et al. American Society of Anesthesiologists consensus-based guidance on preoperative management of patients (adults and children) on glucagon-like peptide-1 (GLP1) receptor agonists. American Society of Anesthesiologists. June 29, 2023. Available at: https://bit.ly/46roAjn. Accessed November 9, 2023.
- Z Bloomgarden. Should glucagon-like peptide-1 receptor agonist treatment be withheld in preoperative management? J Diabetes 2023; 15:712. doi:10.1111/1753-0407.13469