ISSUE1687
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Review the influenza vaccines available in the US for the 2023-2024 season and discuss which vaccines are appropriate for specific patient populations.
- Discuss the risks and benefits associated with seasonal vaccination against influenza.
- Annual vaccination against influenza A and B viruses is recommended for everyone in the US ≥6 months old without a contraindication.
- Vaccination should ideally be offered in September or October and continue to be offered as long as influenza viruses are circulating in the community.
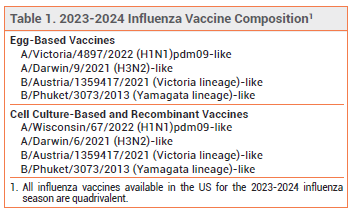
- All influenza vaccines available in the US this season are quadrivalent; they contain two influenza A and two influenza B virus antigens.
- Influenza vaccination reduces the incidence of laboratory-confirmed influenza and the risk of serious complications and death associated with influenza illness.
- In adults ≥65 years old, use of a high-dose, adjuvanted, or recombinant vaccine can improve immune responses and is preferentially recommended over other influenza vaccines.
- Pregnant women should be vaccinated against influenza.
- The ACIP states that persons with a history of egg allergy can receive any age-appropriate influenza vaccine. Additional safety measures are no longer recommended for this patient population.
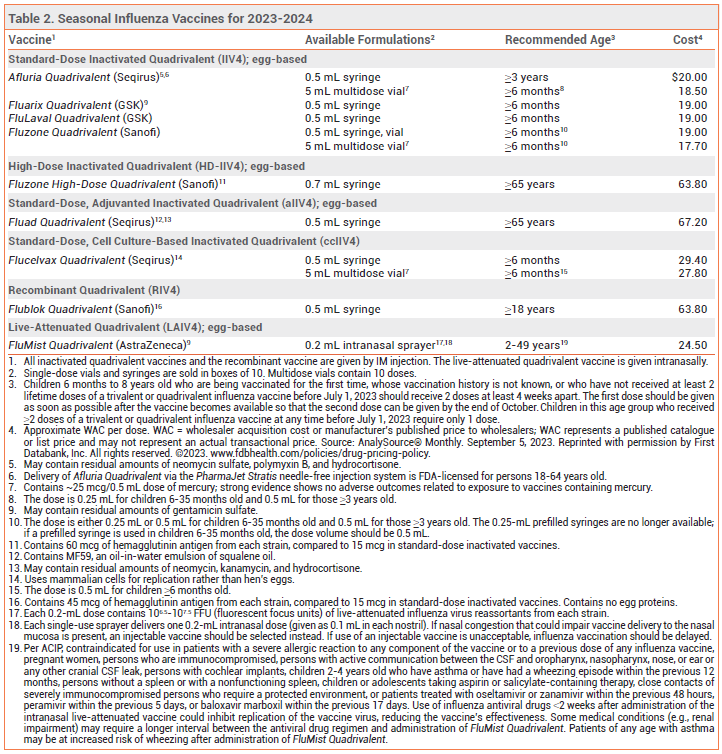
Annual vaccination in the US against influenza A and B viruses is recommended for everyone ≥6 months old without a contraindication.1,2 Influenza vaccines that are available in the US for the 2023-2024 season are listed in Table 2.
COMPOSITION — All influenza vaccines available in the US this season are quadrivalent; they contain two influenza A and two influenza B virus antigens (see Table 1). Influenza A viruses are the main cause of influenza-related morbidity and mortality, especially in older adults. Illness caused by influenza B viruses is usually more severe in children, especially in those <5 years old.3
TIMING — In the US, vaccination against influenza should ideally be offered in September or October and continue to be offered as long as influenza viruses are circulating in the community. In most adults, serum antibody levels peak 1-2 weeks after vaccination. Early vaccination (i.e., in July or August) may result in suboptimal immunity before the end of the influenza season, especially in adults ≥65 years old.4
The Advisory Committee on Immunization Practices (ACIP) recommends that pregnant women who are in their first or second trimester during July or August wait until September or October to be vaccinated to ensure that their babies are protected throughout the influenza season, unless vaccination later is not possible; vaccination during July or August can be considered for women who are in their third trimester.1
Children aged 6 months through 8 years who require 2 doses should receive the first dose as early as possible so that the second dose can be given by the end of October; children in this age group who previously received two doses of an influenza vaccine will only need one dose this season (see Table 2, footnote 3). Vaccination in September or October is preferred for children of any age who need only one dose, but administration in July or August can be considered.
Vaccination of persons with moderate or severe COVID-19 should be postponed until after recovery from acute illness.1
EFFECTIVENESS — Influenza vaccination reduces the incidence of laboratory-confirmed influenza and the risk of serious complications and death associated with influenza illness.5-8 The effectiveness of the seasonal influenza vaccine in preventing influenza illness depends on several factors, including the match between the vaccine and circulating strains and the immunologic response of the recipient. Vaccine effectiveness is greatest when the match is close, but even when it is suboptimal, vaccination can still substantially reduce the risk of influenza-related hospitalization and death.9-11 During the curent influenza season in the Southern Hemisphere, vaccination was associated with a 52% reduction in the risk of influenza-associated hospitalizations.12
OLDER ADULTS — Older adults are at increased risk for severe influenza-associated illness, hospitalization, and death. They may have weaker immunogenic responses to influenza vaccination than younger persons, and their antibody levels may decline more rapidly, decreasing vaccine effectiveness.13
In a cohort study of hospitalized adults ≥60 years old with cardiovascular disease, influenza vaccination was associated with a reduced risk of in-hospital death, recurrent hospitalization for ischemic heart disease, and respiratory hospitalization during the influenza season.14
High-Dose Vaccine – Fluzone High-Dose Quadrivalent, an inactivated vaccine that contains 4 times the amount of antigen included in standard-dose inactivated influenza vaccines, is FDA-licensed for use in persons ≥65 years old.
In a randomized, double-blind trial in 31,989 adults ≥65 years old during 2 influenza seasons, a high-dose inactivated trivalent vaccine (Fluzone High-Dose; no longer available) induced significantly greater antibody responses than a standard-dose inactivated trivalent vaccine and was 24% more effective in preventing laboratory-confirmed influenza illness.15 In observational studies and randomized trials in adults ≥65 years old, use of a high-dose inactivated trivalent or quadrivalent vaccine was associated with a reduced risk of respiratory-related and all-cause hospitalization and death compared to standard-dose inactivated trivalent or quadrivalent vaccines.16-20
In a randomized trial in 5260 patients (mean age 65.5 years) with high-risk cardiovascular disease, use of a high-dose inactivated trivalent vaccine over 3 influenza seasons did not significantly reduce all-cause mortality or cardiopulmonary hospitalizations compared to standard-dose inactivated quadrivalent vaccines.21
Adjuvanted Vaccine – Fluad Quadrivalent, an adjuvanted inactivated influenza vaccine, is FDA-licensed for use in persons ≥65 years old. It contains MF59, an oil-in-water emulsion of squalene oil that increases the immune response by recruiting antigen-presenting cells to the injection site and promoting uptake of influenza virus antigens.
In a randomized trial in 7082 adults ≥65 years old, an adjuvanted inactivated trivalent vaccine (Fluad; no longer available) elicited significantly greater antibody responses than a nonadjuvanted, standard-dose inactivated trivalent vaccine.22 In observational studies and randomized trials, older adults who received an adjuvanted inactivated trivalent vaccine were less likely to develop symptomatic influenza illness or be hospitalized for influenza or pneumonia than those who received a nonadjuvanted, standard-dose inactivated trivalent vaccine.23-25
Recombinant Vaccine – Flublok Quadrivalent, a recombinant influenza vaccine produced without use of influenza virus or chicken eggs, contains 3 times the amount of antigen included in standard-dose inactivated influenza vaccines. It is FDA-licensed for use in persons ≥18 years old.
In a retrospective cohort study in 12.7 million adults ≥65 years old vaccinated during the 2019-2020 influenza season, the recombinant quadrivalent vaccine was more effective in preventing hospital visits than a nonadjuvanted, standard-dose inactivated quadrivalent vaccine, an adjuvanted trivalent vaccine, and a high-dose trivalent vaccine.26
Choice of Vaccine – In a recent trial in community-dwelling adults 65-82 years old, high-dose, adjuvanted, and recombinant influenza vaccines improved humoral and cell-mediated immune responses compared to standard-dose inactivated vaccines.27 Few trials have directly compared the high-dose, adjuvanted, and recombinant vaccines in older adults and none have shown that any one vaccine is superior to another.
The ACIP preferentially recommends use of a high-dose, adjuvanted, or recombinant influenza vaccine over other available age-appropriate influenza vaccines in adults ≥65 years old; if none of these vaccines are available, any age-appropriate influenza vaccine should be given.1
PREGNANCY — Vaccination protects pregnant women against influenza-associated illness, which can be especially severe during pregnancy, and protects their infants for up to 6 months after birth (influenza vaccines are not approved for use in infants <6 months old).28
The ACIP and the American College of Obstetricians and Gynecologists (ACOG) recommend that pregnant women be vaccinated against influenza (see Timing section).29,30 Pregnant women can receive any age-appropriate inactivated or recombinant influenza vaccine; the live-attenuated intranasal vaccine (FluMist Quadrivalent) should not be used during pregnancy.
Most studies have not found an association between influenza vaccination and adverse pregnancy outcomes, but data demonstrating the safety of vaccination during the first trimester are limited.31 In one prospective study in women who were planning on becoming pregnant or who were pregnant, exposure to an influenza vaccine before or during pregnancy was not associated with an increased rate of miscarriage.32
EGG ALLERGY — The recombinant vaccine (Flublok Quadrivalent) and the cell culture-based inactivated vaccine (Flucelvax Quadrivalent) do not contain egg protein. Other available influenza vaccines may contain trace amounts of egg protein (ovalbumin), but numerous studies have found that patients with a history of egg allergy are not at increased risk for a reaction to influenza vaccines that are propagated in eggs.33
The ACIP, the American Academy of Pediatrics (AAP), and the Joint Task Force on Practice Parameters of the American Academy of Allergy Asthma and Immunology and the American College of Allergy Asthma and Immunology state that persons with a history of egg allergy of any severity can receive any egg- or nonegg-based age-appropriate influenza vaccine without the need for additional safety measures.1,2,34
The American Academy of Pediatrics adds that it is not necessary to inquire about egg allergy before administration of any influenza vaccine, including on screening forms.2
IMMUNOCOMPROMISED PERSONS — The live-attenuated intranasal influenza vaccine should not be used in immunocompromised persons. Inactivated and recombinant vaccines are generally considered safe for use in such persons, but the immune response may be reduced. In two randomized trials in solid-organ transplant recipients, the high-dose vaccine induced significantly greater immune responses than standard-dose vaccines.35,36 Separating the time of influenza vaccination from that of an immunocompromising intervention might be considered.
USE WITH OTHER VACCINES — Any influenza vaccine can be given at the same time as a COVID-19 vaccine, but the vaccines should be administered in separate arms. The ACIP states that coadministration of a respiratory syncytial virus (RSV) vaccine with other adult vaccines during the same visit is acceptable; RSV and influenza antibody titer levels are somewhat lower with coadministration of a quadrivalent seasonal influenza vaccine and a RSV vaccine than with sequential administration (~1 month apart).37
Inactivated and recombinant influenza vaccines can be administered concomitantly or sequentially with live or other inactivated or recombinant vaccines. The live-attenuated intranasal influenza vaccine can be given simultaneously with inactivated or other live vaccines; other live vaccines not administered simultaneously should be given at least 4 weeks later. Because of limited safety data on concurrent use of 2 or more adjuvanted vaccines, use of a nonadjuvanted influenza vaccine may be considered when another adjuvanted vaccine (e.g., Shingrix, Heplisav-B) is going to be administered concurrently.
USE WITH INFLUENZA ANTIVIRALS — Any inactivated or recombinant influenza vaccine can be administered to persons receiving antiviral drugs for treatment or chemoprophylaxis of influenza. Use of oseltamivir (Tamiflu, and generics) or zanamivir (Relenza) within 48 hours before, peramivir (Rapivab) within 5 days before, or baloxavir marboxil (Xofluza) within 17 days before administration of the live-attenuated intranasal influenza vaccine could inhibit replication of the vaccine virus, reducing the vaccine's effectiveness. Persons who receive any of these antiviral drugs during these specified times and through 2 weeks after administration of the live-attenuated vaccine should be revaccinated with an inactivated or recombinant influenza vaccine.
ADVERSE EFFECTS — Influenza vaccination has been associated with Guillain-Barré syndrome, but the absolute risk is very low (about 1-2 additional cases per million persons vaccinated), and influenza infection itself has been associated with the syndrome (about 17 cases per one million patients hospitalized with influenza).38 In a prospective cohort study in patients with diabetes, influenza vaccination was associated with hyperglycemia, but serum glucose levels returned to baseline 2 days after vaccination.39
Except for soreness at the injection site, adverse reactions to inactivated influenza vaccines are uncommon. In clinical trials, a high-dose vaccine (Fluzone High-Dose trivalent) caused more injection-site reactions than standard-dose influenza vaccines. Pain and tenderness at the injection site also occurred more frequently with an adjuvanted vaccine (Fluad trivalent) than with a nonadjuvanted vaccine. Delivery of Afluria by needle-free jet injector has resulted in more mild to moderate local reactions than delivery by standard needle and syringe.
The most common adverse reactions associated with the live-attenuated intranasal vaccine are runny nose, nasal congestion, fever, and sore throat. The vaccine may increase the risk of wheezing, especially in children <5 years old with recurrent wheezing and in persons of any age with asthma. However, a recent study in 151 children with asthma found that the frequency of asthma exacerbations and asthma-related symptoms was similar with the live-attenuated influenza vaccine and a quadrivalent inactivated influenza vaccine.40 Persons who receive the live-attenuated vaccine may shed the vaccine-strain virus for a few days after vaccination, but person-to-person transmission has been rare, and serious illness resulting from transmission has not been reported. Nevertheless, the ACIP states that persons who care for severely immunocompromised patients in protected environments should not receive the live-attenuated vaccine or should avoid contact with such patients for 7 days after receiving it.
INVESTIGATIONAL VACCINES ― Vaccines that provide universal protection against all influenza strains are in development. A vaccine designed to protect against influenza, COVID-19, and respiratory syncytial virus, the three primary causes of viral respiratory disease in older adults, is in development.
- LA. Grohskopf et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices — United States, 2023–24 influenza season. MMWR Recomm Rep 2023; 72:1. doi:10.15585/mmwr.rr7202a1
- Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2023-2024. Pediatrics 2023 Aug 29 (epub). doi:10.1542/peds.2023-063773
- YR Bhat. Influenza B infections in children: a review. World J Clin Pediatr 2020; 9:44. doi:10.5409/wjcp.v9.i3.44
- JM Ferdinands et al. Waning vaccine effectiveness against influenza-associated hospitalizations among adults, 2015-2016 to 2018-2019, United States Hospitalized Adult Influenza Vaccine Effectiveness Network. Clin Infect Dis 2021; 73:726. doi:10.1093/cid/ciab045
- C Arriola et al. Influenza vaccination modifies disease severity among community-dwelling adults hospitalized with influenza. Clin Infect Dis 2017; 65:1289. doi:10.1093/cid/cix468
- B Flannery et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics 2017; 139:e20164244. doi:10.1542/peds.2016-4244
- AP Campbell et al. Effect of vaccination on preventing influenza-associated hospitalizations among children during a severe season associated with B/Victoria viruses, 2019-2020. Clin Infect Dis 2021; 73:e947. doi:10.1093/cid/ciab060
- S Garg et al. 898. Influenza vaccination reduces risk of severe outcomes among adults hospitalized with influenza A(H1N1) pdm09, FluSurv-NET, 2013–2018. Open Forum Infect Dis 2019; 6(Suppl 2):S26. doi:10.1093%2Fofid%2Fofz359.057
- M Darvishian et al. Effectiveness of seasonal influenza vaccine in community-dwelling elderly people: a meta-analysis of test-negative design case-control studies. Lancet Infect Dis 2014; 14:1228. doi:10.1016/s1473-3099(14)70960-0
- MA Rolfes et al. Effects of influenza vaccination in the United States during the 2017-2018 influenza season. Clin Infect Dis 2019; 69:1845. doi:10.1093/cid/ciz075
- LC Sahni et al. Sustained within-season vaccine effectiveness against influenza-associated hospitalization in children: evidence from the New Vaccine Surveillance Network, 2015-2016 through 2019-2020. Clin Infect Dis 2023; 76:e1031. doi:10.1093/cid/ciac577
- AL Fowlkes et al. Interim effectiveness estimates of 2023 Southern Hemisphere influenza vaccines in preventing influenza-associated hospitalizations – REVELAC-i Network, March-July 2023. MMWR Morb Mortal Wkly Rep 2023; 72:1010. doi:10.15585/mmwr.mm7237e1
- B Young et al. Do antibody responses to the influenza vaccine persist year-round in the elderly? A systematic review and meta-analysis. Vaccine 2017; 35:212. doi:10.1016/j.vaccine.2016.11.013
- Y Pang et al. Effectiveness of influenza vaccination on in-hospital death and recurrent hospitalization in older adults with cardiovascular diseases. Int J Infect Dis 2022; 122:162. doi:10.1016/j.ijid.2022.05.059
- CA DiazGranados et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635. doi:10.1056/nejmoa1315727
- S Gravenstein et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir Med 2017; 5:738. doi:10.1016/s2213-2600(17)30235-7
- DK Shay et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012-2013 and 2013-2014. J Infect Dis 2017; 215:510. doi:10.1093/infdis/jiw641
- JKH Lee et al. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: an updated systematic review and meta-analysis. Vaccine 2021; 39(Suppl 1):A24. doi:10.1016/j.vaccine.2020.09.004
- Y Young-Xu et al. High-dose influenza vaccination and mortality among predominantly male, white, senior veterans, United States, 2012/13 to 2014/2015. Euro Surveill 2020; 25:1900401. doi:10.2807/1560-7917.es.2020.25.19.1900401
- ND Johansen et al. A pragmatic randomized feasibility trial of influenza vaccines. NEJM Evid 2023; 2:1. doi:10.1056/EVIDoa2200206
- O Vardeny et al. Effect of high-dose trivalent vs standard-dose quadrivalent influenza vaccine on mortality or cardiopulmonary hospitalization in patients with high-risk cardiovascular disease: a randomized clinical trial. JAMA 2021; 325:39. doi:10.1001/jama.2020.23649
- SE Frey et al. Comparison of the safety and immunogenicity of an MF59®-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine 2014; 32:5027. doi:10.1016/j.vaccine.2014.07.013
- PG Van Buynder et al. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine 2013; 31:6122. doi:10.1016/j.vaccine.2013.07.059
- F Lapi et al. Adjuvanted versus nonadjuvanted influenza vaccines and risk of hospitalizations for pneumonia and cerebro/cardiovascular events in the elderly. Expert Rev Vaccines 2019; 18:663. doi:10.1080/14760584.2019.1622418
- KW McConeghy et al. Cluster-randomized trial of adjuvanted versus nonadjuvanted trivalent influenza vaccine in 823 U.S. nursing homes. Clin Infect Dis 2021; 73:e4237. doi:10.1093/cid/ciaa1233
- HS Izurieta et al. Comparative effectiveness of influenza vaccines among US Medicare beneficiaries ages 65 years and older during the 2019-2020 season. Clin Infect Dis 2021; 73:e4251. doi:10.1093/cid/ciaa1727
- BJ Cowling et al. Comparative immunogenicity of several enhanced influenza vaccine options for older adults: a randomized, controlled trial. Clin Infect Dis 2020; 71:1704. doi:10.1093/cid/ciz1034
- MG Thompson et al. Influenza vaccine effectiveness in preventing influenza-associated hospitalizations during pregnancy: a multi-country retrospective test negative design study, 2010-2016. Clin Infect Dis 2019; 68:1444. doi:10.1093/cid/ciy737
- CDC. Addressing concerns pregnant people might have about influenza vaccine safety. December 2, 2022. Available at: https://bit.ly/3jDkTyg. Accessed September 28, 2023.
- ACOG Committee Opinion No. 732: influenza vaccination during pregnancy. Obstet Gynecol 2018; 131:e109. doi:10.1097/aog.0000000000002588
- A Mehrabadi et al. Association of maternal influenza vaccination during pregnancy with early childhood health outcomes. JAMA 2021; 325:2285. doi:10.1001/jama.2021.6778
- AK Regan et al. Risk of miscarriage in relation to seasonal influenza vaccination before or during pregnancy. Obstet Gynecol 2023:142:625. doi:10.1097/aog.0000000000005279
- M Greenhawt et al. Administration of influenza vaccines to egg allergic recipients: a practice parameter update 2017. Ann Allergy Asthma Immunol 2018; 120:49. doi:10.1016/j.anai.2017.10.020
- American Academy of Allergy Asthma & Immunology. Egg allergy and the flu vaccine. August 7, 2023. Available at: https://bit.ly/3yV2uUI. Accessed September 28, 2023.
- Y Natori et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66:1698. doi:10.1093/cid/cix1082
- AG L’huillier et al. Cell-mediated immune responses after influenza vaccination of solid organ transplant recipients: secondary outcomes analyses of a randomized controlled trial. J Infect Dis 2020; 221:53. doi:10.1093/infdis/jiz471
- M Melgar et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices – United States, 2023. MMWR Morb Mortal Wkly Rep 2023; 72:793. doi:10.15585/mmwr.mm7229a4
- A Babazadeh et al. Influenza vaccination and Guillian-Barré syndrome: reality or fear. J Transl Int Med 2019; 7:137. doi:10.2478/jtim-2019-0028
- AL Hulsizer et al. Hyperglycemia post-influenza vaccine in patients with diabetes. Ann Pharmacother 2023; 57:51. doi:10.1177/10600280221098101
- AG Sokolow et al. Safety of live attenuated influenza vaccine in children with asthma. Pediatrics 2022; 149:e2021055432. doi:10.1542/peds.2021-055432