ISSUE1660
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Michael Viscusi, Pharm.D., Associate Editor: no disclosure or potential conflict of interest to report
- Discuss the recommended use of the new bivalent COVID-19 vaccine.
The FDA has amended its Emergency Use Authorizations (EUAs) for the mRNA COVID-19 vaccines manufactured by Pfizer/BioNTech (Comirnaty) and Moderna (Spikevax) to permit use of bivalent formulations of the products as a single booster dose in persons ≥12 years old (Pfizer) or ≥18 years old (Moderna) whose most recent COVID-19 vaccine dose was a monovalent product given ≥2 months previously as a booster or for completion of a primary series. The bivalent formulations are not authorized for primary immunization. Monovalent Pfizer and Moderna COVID-19 vaccines are no longer authorized for use as booster doses in persons ≥12 years old.1
THE NEW FORMULATIONS – The bivalent vaccine formulations contain equal amounts of two mRNA strands. One strand, which codes for the spike protein of the original (Wuhan-Hu-1) strain of SARS-CoV-2, is identical to that used in the monovalent vaccines. The other strand codes for the spike protein common to the BA.4 and BA.5 Omicron variants of the virus, which are currently dominant in the US.2
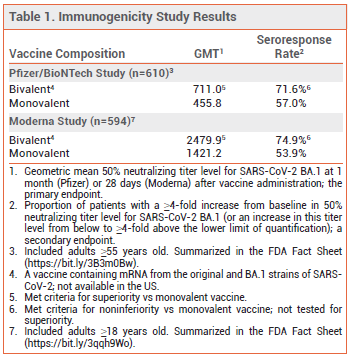
CLINICAL STUDIES – No randomized trials of the new bivalent vaccine formulations are available. Their authorization was based on clinical data with the monovalent products and on the results of randomized immunogenicity studies (summarized in the FDA Fact Sheets) that compared bivalent formulations of the vaccines containing mRNA from the original and BA.1 Omicron strains of SARS-CoV-2 (not authorized in the US) with their respective monovalent formulations.3,4
The immunogenicity studies included 610 adults ≥55 years old (Pfizer) and 594 adults ≥18 years old (Moderna) who had no history of SARS-CoV-2 infection and had previously received two primary-series doses and one booster dose of the applicable monovalent vaccine. Subjects received either a second booster dose of the same monovalent vaccine or a booster dose of the corresponding original/BA.1 bivalent vaccine. In both studies, at ~1 month after the second booster dose, the geometric mean neutralizing antibody titer level and the seroresponse rate against the BA.1 variant were higher with the bivalent vaccine than with the monovalent vaccine (see Table 1); titer levels and seroresponse rates against the original SARS-CoV-2 strain were similar in the two groups.3,4 It is presumed that the newly authorized bivalent formulations of the Pfizer and Moderna vaccines will induce a similar immunogenic response against the BA.4 and BA.5 variants of SARS-CoV-2.
ADVERSE EFFECTS – In the immunogenicity studies, adverse effects of the original/BA.1 bivalent Pfizer and Moderna vaccines were similar to those with the corresponding monovalent products.3,4
DOSAGE AND ADMINISTRATION – CDC guidelines recommend that adolescents and adults who have completed a primary immunization series with any COVID-19 vaccine receive a booster dose of either the bivalent Pfizer vaccine (≥12 years old) or the bivalent Moderna vaccine (≥18 years old) ≥2 months after their most recent monovalent vaccine dose.5 The booster doses of the bivalent vaccines are 30 mcg/0.3 mL for Pfizer and 50 mcg/0.5 mL for Moderna.3,4
- FDA News Release. Coronavirus (COVID-19) update: FDA authorizes Moderna, Pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose. August 31, 2022. Available at: https://bit.ly/3QxSEku. Accessed September 15, 2022.
- CDC. COVID data tracker. Variant proportions. Available at: https://bit.ly/3Ka3HhH. Accessed September 15, 2022.
- FDA. Fact sheet for health care providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA). Pfizer-BioNTech COVID-19 vaccine, bivalent (original and Omicron BA.4/BA.5). Booster dose for 12 years of age and older. August 31, 2022. Available at: https://bit.ly/3B3m0Bw. Accessed September 15, 2022.
- FDA. Fact sheet for health care providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA). Moderna COVID-19 vaccine, bivalent (original and Omicron BA.4/BA.5). Booster dose for 18 years of age and older. August 31, 2022. Available at: https://bit.ly/3qqh9Wo. Accessed September 15, 2022.
- CDC. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. September 2, 2022. Available at: https://bit.ly/3KgPdxl. Accessed September 15, 2022.