ISSUE1646
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Michael Viscusi, Pharm.D., Associate Editor: no disclosure or potential conflict of interest to report
- Review the efficacy and safety of bebtelovimab for treatment of COVID-19.
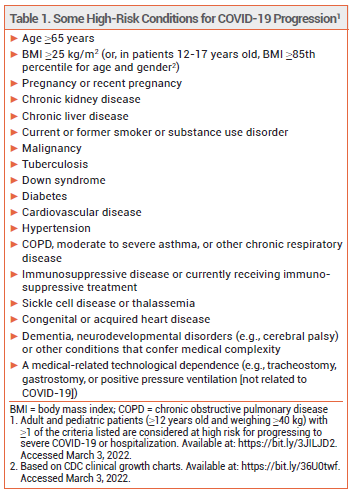
The investigational monoclonal antibody bebtelovimab (LY-CoV1404 – Lilly) has been granted an FDA Emergency Use Authorization (EUA) for IV treatment of mild to moderate COVID-19 in patients ≥12 years old who weigh ≥40 kg and are at high risk of progressing to severe disease, including hospitalization and death, and for whom alternative treatment options are unavailable or inappropriate.1 Bebtelovimab is active against the Omicron variant of SARS-CoV-2; sotrovimab (VIR-7831) is the only other monoclonal antibody currently available for treatment of COVID-19 that is active against Omicron.2
NIH GUIDELINES — Current NIH guidelines recommend that high-risk outpatients with mild to moderate COVID-19 who are ≥12 years old and weigh ≥40 kg receive antiviral treatment with (in order of preference) a 5-day course of oral nirmatrelvir with ritonavir (Paxlovid), a single IV infusion of sotrovimab, or a 3-day course of IV remdesivir (Veklury). Paxlovid and sotrovimab are preferred over remdesivir mainly because of logistical concerns associated with administration of remdesivir on 3 consecutive days. If all three of these drugs are inappropriate or unavailable, use of either a single IV injection of bebtelovimab or (in adults only) a 5-day course of oral molnupiravir is recommended. Molnupiravir is less effective than Paxlovid, sotrovimab, and remdesivir, and clinical data supporting the efficacy of bebtelovimab are limited.3
PHARMACOLOGY — Like other anti-SARS-CoV-2 monoclonal antibodies, bebtelovimab binds to the viral spike protein and prevents its attachment to the human ACE2 receptor. The antibody retains full neutralization activity in vitro against the BA.1 and BA.2 (Omicron) variants of the virus.4 Administration of anti-SARS-CoV-2 antibodies to high-risk outpatients infected with a susceptible viral variant has decreased rates of hospitalization or death due to COVID-19.2,5,6
CLINICAL STUDIES — Authorization of bebtelovimab was based on in vitro neutralization data and the results from three cohorts of an unpublished single-dose, phase 2 clinical trial (BLAZE-4; summarized in the FDA Fact Sheet), which included a total of 706 outpatients with COVID-19 symptoms who had undergone confirmatory testing for SARS-CoV-2 infection within the previous 3 days. BLAZE-4 was completed before the emergence of the Omicron variant.
In one cohort, 380 unvaccinated, mostly low-risk adults were randomized to receive bebtelovimab 175 mg alone, bebtelovimab 175 mg plus bamlanivimab 700 mg and etesevimab 1400 mg, or placebo. (Bamlanivimab and etesevimab are not currently authorized for use in the US because they lack activity against the Omicron variant of SARS-CoV-2.7) A persistently high viral load at day 7, the primary endpoint, was present in 14% of patients who received bebtelovimab alone, 13% of those who received all three antibodies, and 21% of those who received placebo; these differences were not statistically significant. The median time to symptom resolution was shorter with bebtelovimab alone than with placebo (6 days [95% CI 5-7 days] vs 8 days [95% CI 7-9 days]). Hospitalization due to COVID-19 occurred in <3% of patients in all three groups.
In another cohort, 150 mostly high-risk patients were randomized to receive bebtelovimab 175 mg either alone or in combination with bamlanivimab 700 mg and etesevimab 1400 mg. Patients in the two groups had similar rates of hospitalization due to COVID-19 (3% with bebtelovimab alone vs 4% with combination therapy) and reductions in mean viral load. The median time to symptom resolution after administration of bebtelovimab alone was 7 days.
In a single-arm cohort, 176 mostly high-risk patients received bebtelovimab 175 mg, bamlanivimab 700 mg, and etesevimab 1400 mg. Three patients (1.7%) required hospitalization due to COVID-19. The median time to symptom resolution was 8 days.4
ADVERSE EFFECTS — The most common adverse effects of bebtelovimab in clinical trials (frequency <1%) were rash, pruritus, and infusion-related reactions. Hypersensitivity reactions, including anaphylaxis, have occurred rarely with use of anti-SARS-CoV-2 monoclonal antibodies.4
DOSAGE AND ADMINISTRATION — Bebtelovimab is supplied in 175 mg/2 mL vials. The recommended dosage is 175 mg administered by IV injection over at least 30 seconds. Bebtelovimab should be refrigerated during storage, but allowed to sit at room temperature for 20 minutes before it is given. The line used for administration should be flushed with normal saline after the drug is injected.
Bebtelovimab should be given as soon as possible after a positive SARS-CoV-2 test result and within 7 days of symptom onset. Patients should be monitored for at least 1 hour after receiving the drug.4
AVAILABILITY — The US government has purchased 600,000 doses of bebtelovimab to be distributed to healthcare facilities free of charge.8
CONCLUSION — The FDA has issued an Emergency Use Authorization (EUA) for the monoclonal antibody bebtelovimab (LY-CoV1404) for IV treatment of mild to moderate COVID-19 in outpatients at high risk for progression to severe disease. Bebtelovimab retains full activity in vitro against the Omicron variant of SARS-CoV-2. Although clinical data supporting its efficacy are limited, bebtelovimab appears to be safe, and results from trials of other anti-SARS-CoV-2 monoclonal antibodies suggest that it could decrease the risk of hospitalization and death. Until more data become available, however, bebtelovimab should only be used when other treatment options for COVID-19 are unavailable or inappropriate.
- FDA News Release. Coronavirus (COVID-19) update: FDA authorizes new monoclonal antibody for treatment of COVID-19 that retains activity against omicron variant. February 11, 2022. Available at: https://bit.ly/3gMLn0L. Accessed March 3, 2022.
- An EUA for sotrovimab for treatment of COVID-19. Med Lett Drugs Ther 2021; 63:97.
- NIH. The COVID-19 Treatment Guidelines Panel’s statement on the role of bebtelovimab for the treatment of high-risk, nonhospitalized patients with mild to moderate COVID-19. March 2, 2022. Available at: https://bit.ly/35OhB9S. Accessed March 3, 2022.
- FDA. Fact sheet for health care providers. Emergency Use Authorization for bebtelovimab. February 11, 2022. Available at: https://bit.ly/3HO6goe. Accessed March 3, 2022.
- An EUA for bamlanivimab and etesevimab for COVID-19. Med Lett Drugs Ther 2021; 63:49.
- An EUA for casirivimab and imdevimab for COVID-19. Med Lett Drugs Ther 2020; 62:201.
- FDA Statement. Coronavirus (COVID-19) update: FDA limits use of certain monoclonal antibodies to treat COVID-19 due to the omicron variant. January 24, 2022. Available at: https://bit.ly/3GVGsWR. Accessed March 3, 2022.
- HHS. Secretary Becerra announces HHS purchase of 600,000 treatment courses of new monoclonal antibody that works against Omicron. February 10, 2022. Available at: https://bit.ly/36lZoRf. Accessed March 3, 2022.
Additional Content Available Online: COVID-19 Tables/Charts
See the latest information on COVID-19, including our continuously updated tables/charts on treatments, vaccines, and dosing recommendations.