ISSUE1562
Acute cough (<3 weeks in duration) generally does not require pharmacologic treatment, especially in children. Suppression of productive cough may be harmful.1 Management of patients with cough should include elimination of any precipitating factor (e.g., cigarette smoking) and treatment of any underlying cause such as upper airway cough syndrome, gastroesophageal reflux disease, asthma, or other pulmonary disease.
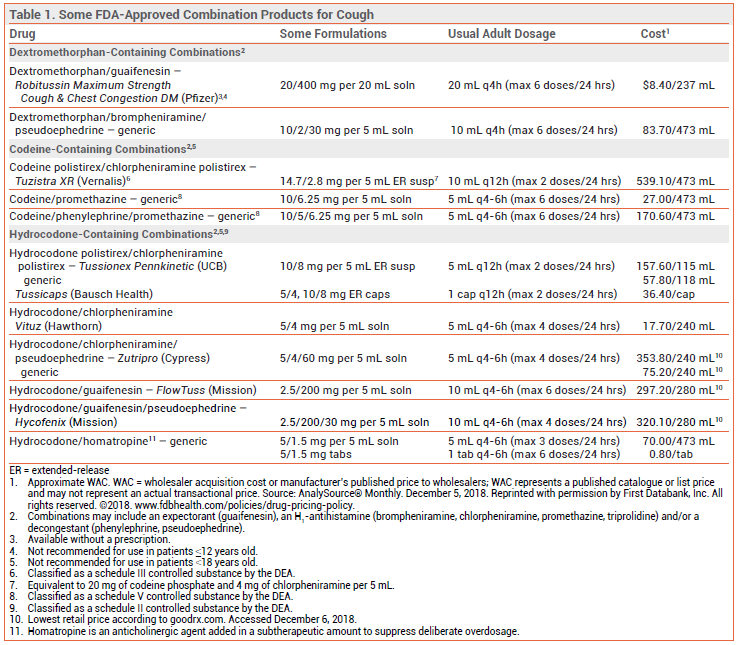
Cough suppressants are widely available over the counter (OTC) and by prescription, often in combination with an expectorant, an H1-antihistamine, and/or a decongestant.2,3
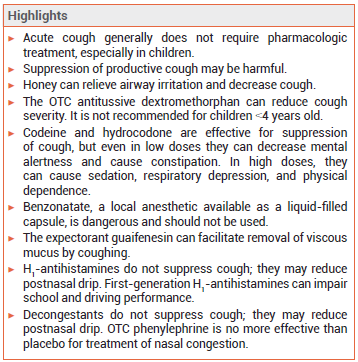
HONEY — A single nocturnal dose (10 g) of honey can relieve airway irritation and decrease cough.4 In comparative studies, honey has been about as effective in suppressing cough as dextromethorphan.3 It should not be used in children <1 year old because it contains spores of Clostridium botulinum that can cause infantile botulism.
DEXTROMETHORPHAN — An OTC derivative of morphine with no analgesic or addictive properties at recommended doses, dextromethorphan can reduce cough severity. Clinical trials comparing it with codeine for treatment of chronic cough have produced conflicting results. Dextromethorphan has not been shown to be effective or safe in young children.1,5
Adverse Effects – Dextromethorphan can cause confusion, excitement, irritability, nervousness, and, in high doses, nausea, vomiting, and headache. Extremely high doses of dextromethorphan can cause euphoria and dissociative effects.
CODEINE AND HYDROCODONE — The opioid agonists codeine and hydrocodone are effective in suppressing cough. Codeine is a weak opioid agonist that must be converted by CYP2D6 to morphine for most of its analgesic and antitussive effects. About 10% of the population are CYP2D6 poor metabolizers; for them, codeine may be ineffective. CYP2D6 ultra-rapid metabolizers may be at greater risk for toxicity. Opioid-containing cough products are generally available only by prescription in the US (a few states and some other countries, including Canada, permit OTC sale of some codeine products).
Adverse Effects – Even in low doses, codeine and hydrocodone can decrease mental alertness and cause constipation. In high doses, they can cause sedation, respiratory depression, and physical dependence. The FDA recently required changes in the labeling of all cough and cold products that contain codeine or hydrocodone because of concerns about the risk of respiratory depression and death in children; these products are no longer indicated for use in patients <18 years old.6 Codeine/promethazine cough syrup, often in combination with soda, candy, or alcohol, has become a recreational drug of choice among young adults.
BENZONATATE (Tessalon Perles, and generics) — A polyglycol derivative structurally related to the local anesthetics procaine and tetracaine, benzonatate is a prescription alternative to opioids for suppression of severe cough. It suppresses the cough reflex by anesthetizing stretch receptors in the lower respiratory tract. Benzonatate is available as a liquid-filled capsule, which must be swallowed whole. It is not indicated for use in children <10 years old.
Adverse Effects – Benzonatate can cause nausea, dizziness, headache, sedation, a feeling of numbness in the chest, mental confusion, and visual hallucinations. Chewing or sucking the liquid-filled capsules, which look like candy, can cause laryngospasm, bronchospasm, and circulatory collapse. Ingestion of a small handful of capsules has caused seizures, cardiac arrhythmias, and death in adults.7 The FDA has warned that taking even a single capsule can be fatal for young children.8 The risks associated with use of benzonatate clearly outweigh its benefits.
GUAIFENESIN — The expectorant guaifenesin increases the volume of secretions in the respiratory tract, facilitating removal of viscous mucus by coughing. Clinical trials comparing it to placebo for treatment of cough have produced conflicting results.9 Guaifenesin is available by prescription in combination with an opioid, and OTC both alone and in combination preparations. Guaifenesin extended-release tablets are not recommended for use in children <12 years old.
Adverse Effects – In recommended doses, guaifenesin is generally considered safe. High doses can cause dizziness, drowsiness, headache, nausea, and vomiting. Continued use of high doses has been associated with development of kidney stones.
ANTIHISTAMINES — H1-antihistamines do not suppress cough. Their antimuscarinic activity might reduce coughing in some patients by reducing postnasal drip.10 They are available in many combination products that include an antitussive.
Adverse Effects – First-generation H1-antihistamines such as diphenhydramine can cause dry mouth, dry eyes, blurred vision, constipation, bladder outflow obstruction leading to urinary retention, and CNS impairment with or without sedation. They can interfere with learning and memory, impair performance on school examinations, decrease work productivity, and increase the risk of on-the-job accidents. When these drugs are taken at night, adverse effects on wakefulness and psychomotor performance can persist the next day. A single 50-mg dose of diphenhydramine caused more errors on a driving simulation test than a blood alcohol level of 0.1%.11
DECONGESTANTS — Decongestants do not directly suppress cough, but they may help reduce coughing by reducing postnasal drip. They are often available OTC in combination products that include an antitussive. Decongestants are not recommended for use in children <4 years old and they should not be used with or within 14 days of a monoamine oxidase (MAO) inhibitor.
OTC phenylephrine is no more effective than placebo for treatment of nasal congestion.12 Access to products containing pseudoephedrine or ephedrine is restricted because of their potential for abuse: they are kept behind the pharmacy counter, a valid photo ID is required, and there is a limit to the amount that can be purchased in a single day or in a 30-day period. Phenylpropanolamine is no longer available in the US for human use because it was associated with an increased risk of hemorrhagic stroke in women who took it as an appetite suppressant.
Adverse Effects – Oral decongestants increase heart rate and blood pressure. They can cause transient excitability, insomnia, headache, nervousness, confusion, dizziness, nausea, and urinary retention. Dry nose and throat and rebound nasal congestion can occur.
SHORT-ACTING BRONCHODILATORS — The short-acting beta2-adrenergic agonist (SABA) albuterol (Proventil HFA, and others) and the antimuscarinic ipratropium (Atrovent HFA, and others) have been used alone and in combination to treat cough that has a bronchospastic component.
Adverse Effects – Inhaled SABAs can cause paradoxical bronchospasm, tremor, tachycardia, QT interval prolongation, hyperglycemia, hypokalemia, and hypomagnesemia (especially if used in high doses).
Ipratropium can cause dry mouth, pharyngeal irritation, increased intraocular pressure, and urinary retention.
CORTICOSTEROIDS — Oral and inhaled corticosteroids have been used for treatment of cough in patients without asthma. In a randomized trial in 401 nonasthmatic adults with acute lower respiratory infection, oral prednisolone was not significantly more effective than placebo in reducing the duration of cough or cough severity.13 Controlled trials of inhaled corticosteroids for suppression of cough unrelated to asthma have produced conflicting results.14,15
Adverse Effects – Local adverse effects of inhaled corticosteroids include oral candidiasis (thrush), dysphonia, and reflex cough and bronchospasm. Clinically relevant effects on hypothalamic-pituitary-adrenal (HPA) axis function generally do not occur with low- or medium-dose inhaled corticosteroids.
- MA Malesker et al. Pharmacologic and nonpharmacologic treatment for acute cough associated with the common cold. Chest 2017; 152:1021.
- JA Lowry et al. Over-the-counter medications: update on cough and cold preparations. Pediatr Rev 2015; 36:286.
- M Weinberger and L Hendeles. Nonprescription medications for respiratory symptoms: facts and marketing fictions. Allergy Asthma Proc 2018; 39:169.
- HA Cohen et al. Effect of honey on nocturnal cough and sleep quality: a double-blind, randomized, placebo-controlled study. Pediatrics 2012; 130:465.
- WS Yancy et al. Efficacy and tolerability of treatments for chronic cough: a systematic review and meta-analysis. Chest 2013; 144:1827.
- FDA drug safety communication: FDA requires labeling changes for prescription opioid cough and cold medicines to limit their use to adults 18 years and older. Available at: www.fda.gov. Accessed December 6, 2018.
- SC Bishop-Freeman et al. Benzonatate toxicity: nothing to cough at. J Anal Toxicol 2017; 41:461.
- MW McLawhorn et al. Analysis of benzonatate overdoses among adults and children from 1969-2010 by the United States Food and Drug Administration. Pharmacotherapy 2013; 33:38.
- SM Smith et al. Over-the-counter (OTC) medications for acute cough in children and adults in community settings. Cochrane Database Syst Rev 2012; 8:CD001831.
- PV Dicpinigaitis et al. Inhibition of cough reflex sensitivity by diphenhydramine during acute viral respiratory tract infection. Int J Clin Pharm 2015; 37:471.
- JM Weiler et al. Effects of fexofenadine, diphenhydramine, and alcohol on driving performance. A randomized, placebo-controlled trial in the Iowa driving simulator. Ann Intern Med 2000; 132:354.
- In brief: Oral phenylephrine for nasal congestion. Med Lett Drugs Ther 2015; 57:174.
- AD Hay et al. Effect of oral prednisolone on symptom duration and severity in nonasthmatic adults with acute lower respiratory tract infection: a randomized clinical trial. JAMA 2017; 318:721.
- M El-Gohary et al. Corticosteroids for acute and subacute cough following respiratory tract infection: a systematic review. Fam Pract 2013; 30:492.
- KJ Johnstone et al. Inhaled corticosteroids for subacute and chronic cough in adults. Cochrane Database Syst Rev 2013; 3:CD009305.